Recent healthcare modelling based on published data has estimated 653 000 healthcare-associated infections (HAIs) annually among adult inpatients in English NHS hospitals, with 22 800 annual deaths (Guest et al, 2020). Collectively, the cost to the NHS in England of these challenging numbers is a staggering £2.7 billion. Bloodstream infections represent an estimated 7.3% of the total HAIs (Guest et al, 2020) with as many as 70% of catheter-associated bloodstream infections (CABSIs) thought to be preventable (Umscheid et al, 2011). Peripheral intravenous catheters (PIVCs) are the most frequently used invasive devices in hospitals with estimates as high as 70% of all inpatients requiring a PIVC during their stay in the hospital environment (Zingg et al, 2009). Based on NHS Supply Chain data, more than 300 English NHS trusts purchase more than 25 million safety peripheral intravenous catheters (SPIVCs) annually (NHS Clinical Evaluation Team, 2018).
Growing concern for PIVC care
Notwithstanding concern regarding unnecessary insertion, recent research has highlighted the under-reported risks posed by the placement and management of PIVCs including inadequate technique for skin antisepsis (Zhang et al, 2016; Mermel, 2017; Saliba et al 2018; Blanco-Mavillard et al, 2019; Høvik, 2019).
Although superficially PIVC infections look to be smaller in number than central venous access device (CVAD) infections, considerably more PIVCs are inserted overall compared with CVADs and absolute infection rates are in fact similar for both device types (Zhang et al, 2016; Sato et al, 2017). Moreover, there is a high rate of reported PIVC failure (Helm et al, 2015). In the state of Pennsylvania in the USA, most untypically, hospitals are required to report all laboratory-confirmed bloodstream infections (LCBIs), not solely the more typical central-line associated bloodstream infections (CLABSIs). Data from 2011-2012 highlighted the large, and increasing, numbers of line bacteraemia that were not CLABSIs, raising the question: how many were actually PIVC infections (Davis, 2014)? There is nothing to suggest that this might not be a typical incidence, when reported, and raises a question of effective surveillance. All these authors, and others, highlighted that to improve patient safety, there is a compelling need to focus anew on PIVC insertion and maintenance (Trinh et al, 2011).
As the risks of PIVC infection are better recognised there has been a transition towards longer PIVC dwell times. For some years, dwell times have remained within predefined time frames, varying between 24 hours and 96 hours. However, the advent of ‘clinical indication’, based on best evidence, recommends the removal of PIVCs based on site and device integrity—notably, this is open-ended (Van Donk et al, 2009; Rickard et al, 2010; Webster et al, 2019). This presents both cause for potential concern and opportunity for improvement. In other words, although this evidence-based approach to PIVC removal is welcomed, it also highlights the need for improved standards of PIVC insertion, maintenance and surveillance, and offers scope for innovation.
Skin and skin flora in context of PIVCs
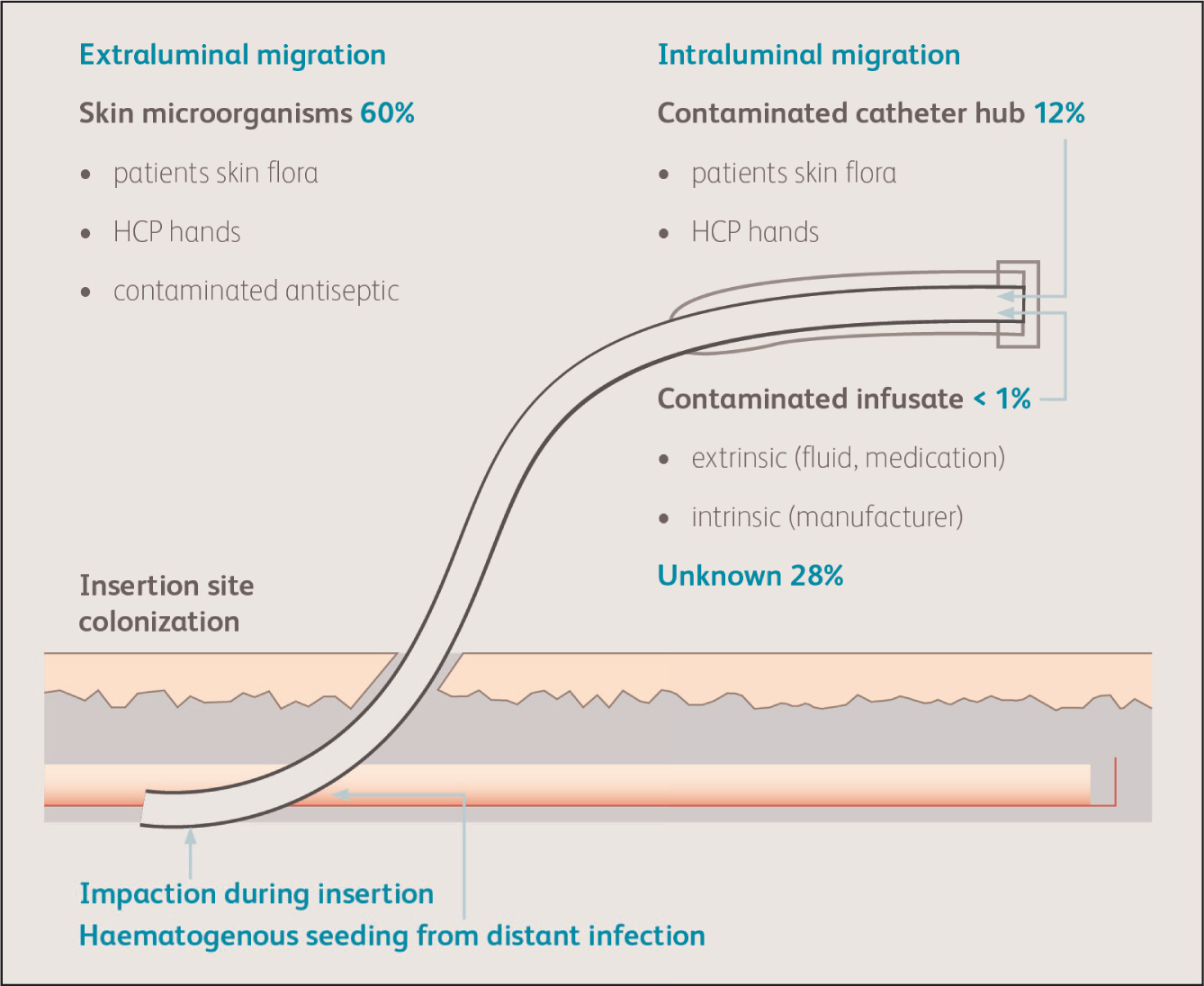
Human adult skin is approximately two square metres in area and weights around 3.6 kg. This important organ is a primary defence against pathogenic microorganisms. Its structure and purposes are well understood; however, perhaps less understood is the microbiology of skin flora and the potential for infection when breached or disturbed by PIVC access. An area of around 1 cm2 of skin can host as many as 10 million aerobic bacteria, which are a leading cause of HAIs (Hibbard, 2005). Importantly, because approximately 80% of skin flora resides in the first five cell layers of skin (Brown, 1989), when the skin is incised or cannulated, the exposed tissue is at risk of contamination, which can lead to extraluminal microorganism migration through the created ‘wound’, potentially causing infection, and secondary biofilm development caused by intraluminal migration later in the process (Zhang et al, 2016). Each and every time a vascular access device (VAD) is inserted, accessed or managed (applying or changing a PIVC dressing), failures in aseptic technique leave the patient vulnerable to microbial migration from both their own skin and the healthcare environment (the health professional, the physical environment and the air environment).
In this light, the skin is both an important protector and protagonist in prevention of healthcare-associated infections (HAIs). In the USA, it has been estimated that 60% of CABSIs are caused by micro-organisms from the patient's skin (Safdar and Maki, 2004; Maki et al, 2006) (Figure 1). Numerous microorganisms are considered a part of the normal human skin flora, including Staphylococcus epidermidis and Staphylococcus aureus. It is worth noting that PIVCs are one of the most common sources of Staphylococcusaureus bacteraemia (Blauw et al, 2019).
Best practice in skin antisepsis
Effective skin antisepsis (see Box 1) plays a critical role in protecting patients from infections during invasive clinical procedures, particularly during the placement of indwelling VADs (Zhang et al, 2016). To mitigate risk, best practice can be defined as ‘quality care deemed optimal based upon a prevailing standard described by evidence’ (Nelson, 2014). For skin antisepsis, best practice includes an appropriate application technique (Casey et al, 2017), a time period for application and complete drying (Tepus et al, 2008; Silva, 2014), plus actions and methodologies to minimise the risk of introducing harmful microorganisms (Rowley et al, 2010) into the procedure.
Best practice naturally includes an appraisal of the most effective disinfection solution and method of application. In recent years, based on best evidence, clinical guidelines have increasingly advised the use of chlorhexidine gluconate in combination with isopropyl alcohol as being most effective in reducing CABSIs (Loveday et al, 2014; Mimoz et al, 2015; Gorski et al, 2016).
Probably reflecting the robust constitution of the skin and its various layers, it would seem that the manner of application of skin disinfectant is significant too. A ‘cross-hatching’, back- and-forth disinfecting technique is considered to be 10 times more effective at reducing bacterial load than the traditional so-called concentric circle technique for skin antisepsis (McDonald et al, 2001). It enables maximum contact between the skin and antiseptic, and helps the solution to reach and disinfect deeper cell layers of the skin (Silva, 2014). Traditionally, the concentric circle technique has persisted in practice without scientific evidence to warrant its use (Tepus et al, 2008; Hadaway, 2012; Tung, 2013). Therefore, recommended skin antisepsis technique for PIVC insertion is a combination of a ‘cross hatch’, disinfection technique, with 2% chlorhexidine gluconate in 70% isopropyl alcohol, then allowing the site to air dry prior to insertion. For patients with sensitivity to chlorhexidine, povidone iodine in alcohol can be used (Tepus et al, 2008; Hadaway, 2012; Tung, 2013; Loveday et al, 2014; Silva, 2014; Gorski et al, 2016; Casey et al, 2017).
The Association for Safe Aseptic Practice (ASAP) works to support improvement in standards of aseptic technique using the A N T T Aseptic Non Touch Technique, a proprietary comprehensive clinical practice framework for aseptic technique. Fit-for-purpose medical equipment and supplies are naturally a key factor in achieving effective ANTT, or indeed any type of aseptic technique. To this end, the ASAP's work includes supporting industry to help ensure synergy and safety between products and aseptic practice. Medical products can, in fact, bring about significant improvement in aseptic technique when novel designs address problematic human factors. A good example of this has been the advent of passive disinfection for IV hub disinfection that reduced reliance on specific cleaning techniques (Moureau and Flynn, 2015; Gorski et al, 2016). A similar step change has been seen for skin antisepsis with the advent of purpose-designed applicators.
Product types for PIVC skin antisepsis
The two most common product types used to deliver chlorhexidine/isopropyl alcohol skin disinfection for skin PIVC are fibre-based ‘wipes’ and specifically designed for purpose hand-held applicators. Currently, the only single-use applicators that are licensed and commercially available are BD ChloraPrepTM (Beckton, Dickinson, UK) applicators. ChloraPrep is licensed by the Medicines and Healthcare products Regulatory Agency (MHRA), the UK's licensing regulator that evaluates safety and efficacy of medicines and medical devices. ChloraPrep is specifically licensed as a medicinal product intended for preoperative skin antisepsis. There is an assumption that health professionals will adhere to MHRA guidance by using the appropriate licensed medicinal product for preoperative skin antisepsis. MHRA guidance does indeed state that using the appropriately authorised product for its specific intended use is the best way of minimising harm. In practice, MHRA guidance is not to be taken as a complete or definitive statement of the law. As such, there is nothing to restrict a health professional from using non-licensed chlorhexidine products for skin preparation prior to invasive procedures such as the insertion of PIVCs. Of note, there is guidance provided by professional bodies such as the General Medical Council (GMC) and Nursing and Midwifery Council (NMC) on the use of an unlicensed product as a medicine. Despite this, some products that the MHRA would classify as medical devices or even biocides could be, and in some cases are, used for skin antisepsis.
This situation does not necessarily present patient risk for preoperative skin antisepsis, but quite simply, the risks compared with licensed products are unknown. The MHRA, as with similar regulatory bodies in other countries, ensures that products prove their efficacy and safety before patients are exposed to them. When unlicensed products are used for skin antisepsis this regulatory structure is bypassed and healthcare organisations, and not least patients, have no assurance that the product they are using has undergone stringent testing, and will also not have a formal mechanism for surveillance and feedback of any adverse incidents.
Organisations that accept this risk for financial or other rationale typically acknowledge their use of unlicensed products as a medicine on a local risk register. In light of the Mid-Staffordshire Public Inquiry and the call for greater transparency in healthcare organisations (Francis, 2013), one might consider how transparent in actual practice, this approach is to patients. Either way, the MHRA is unequivocal: ‘Where an authorised product exists this should be used in preference to another product…’(MHRA, 2020).
ChloraPrep
ChloraPrep applicators address a number of problematic issues and human factors for skin antisepsis. They contain a sterilised product solution of chlorhexidine gluconate and isopropyl alcohol, addressing the potential negative impact of using non-sterile solution for skin antisepsis (Vigeant et al, 1998; Weber et al, 2007; Leong et al, 2018; Song et al, 2018;, 2019). This risk led the US medicines regulatory body, the Food and Drug Administration (FDA), to make it mandatory for manufacturers to write on the packaging whether the content of a skin antisepsis product is sterile or not (FDA, 2016).
ChloraPrep products contain a defined and visible amount of chlorhexidine/isopropyl alcohol solution with assurance of stability ensuring sufficient fluid volume to counter evaporation and facilitate adequate skin coverage and skin impregnation (Tarka et al, 2019). Different volumes of solution are required for different procedures and ChloraPrep products range from 0.67 ml for peripheral application to 26 ml for surgical procedures. In contrast, wipes typically do not appear to assure a precise volume per wipe, seemingly creating uncertainty in usage.
Wipes can be used with effective non-touch technique; however, the various possible approaches to their use increase potential for human factors and subsequently practice variability. For example, wipes may be used folded, unfolded or ‘scrunched up’. Such variables can impact effective non-touch technique. Wipes also bring the operator's fingers closer to the Key-Site. This is not addressed in product instruction because wipe manufacturers, of course, do not provide detailed instructions for their use for skin antisepsis as this is not their intended or indeed stated function. (They are typically stated for disinfecting medical devices or helping remove adhesive residue from dressings.) Method of use can therefore be unclear and left open to interpretation—for example, how and how much the wipe should be unfolded and how best handled to ensure a non-touch technique.
ChloraPrep applicators are opened ready for use, albeit requiring an action to first release the solution. Hand-held wands then promote non-touch technique by separating the health professional's fingers from the applicator surface and the insertion site, helping to protect Key-Parts and Key-Sites (see Box 1). A wand applicator system allows a better control of the flow rate being delivered onto the skin. There is essentially only one way of holding the applicator, promoting ease of use and less potential for variability. Because intended for PIVC insertion and other procedures, an applicator system is supported by manufacturer's instructions.
Until a wipe is granted marketing authorisation from the MHRA, ChloraPrep as a medicinal product is the only licensed and commercially available applicator for disinfection of the skin prior to skin antisepsis prior to invasive medical procedures in the UK. SeppTM, FreppTM etc received an MHRA marketing authorisation for medical use and drug approval from the US FDA. However, from a small convenience sample of policy and procedure documents from NHS trusts obtained online using a Google search, only 50% (5/10) carried an instruction to use a specific product that is licensed as a medicinal product (identified as either ChloraPrep or [ChloraPrep] Sepp); 30% (3/10) did not mention the type of product, whereas 20% (2/10) specified the use of a (non-licensed) ‘wipe’ for skin antisepsis.
Addressing problems of practice with a bundle approach
Identifying problems with increasing incidence of PIVC infection, a bundle approach was implemented at Methodist Hospitals (Gary, Indiana, USA) based on evidence-based standards of practice (Gorski et al, 2016). The bundle integrated several practice recommendations including the use of ChloraPrep for skin antisepsis. Recognising the lack of surveillance around PIVC insertion and management, the bundle included careful assessment of the insertion site, ongoing surveillance of process and outcomes, and a review in ‘real time’ of any infections on the ward. After 12 months DeVries et al (2016) reported a 37% reduction in primary bacteraemia, a 19% reduction in PVIC-associated bloodstream infections after 24 months and a 75% reduction in CLABSI in the intensive therapy unit; this was coupled with improved patient satisfaction, longer indwell times and significant cost savings from rationalising supplies (DeVries et al, 2016).
The risk of suboptimal skin antisepsis
Registered nurses undergoing PIVC training in a large teaching hospital in England were asked to use and discuss the application of wipes and ChloraPrep. There was group uncertainty whether the wipe was best unfolded, left folded, or ‘scrunched up’. Some users found it difficult to only handle one side of it, with the other side exclusively in contact with the patient's skin (non-touch technique). Towards the end of the 30-second skin cleaning some users felt the wipe was running dry, with the skin site accordingly drying. When using ChloraPrep Sepp, some users commented on the relatively large amount of solution available and easily applied to skin. There was no debate or variance as to the handling of the device, probably due to the simple applicator design. Non-touch technique was achieved naturally by design without the need for any noticeable consideration of handling technique.
Improving practice and outcomes
Procedure-based ‘care bundles’ can help ensure that busy staff have easy access to the most appropriate equipment for any given procedure and, in effect, help direct best practice. When used for PIVC insertion and maintenance they have been shown to reduce PIVC-related bloodstream infections (Mestre et al, 2013; DeVries et al, 2016). ChloraPrep has been reported in several studies of clinical care bundles; notably, Steere and colleagues reporting results from the PIV5-Rights bundle, demonstrated improved single catheter dwell times (89% vs 15%) and reduced failure rates due to complications (11% vs 85%) (Steere et al, 2019).
To ensure patient safety, the various equipment contained in a care bundle or ‘cannulation pack’ requires effective integration with a standard aseptic technique. To this end, the widespread adoption of the proprietary ANTT approach as a standard aseptic technique in the NHS, and a de facto standard internationally (Rowley and Clare, 2020), provides a ready-made educational and clinical practice platform for using care bundles as part of PIVC improvement initiatives. Examples of this greater synergy include the inclusion of the proprietary ANTT as part of clinical care bundles to improve incidence of CRBSI (Mutalib et al, 2015; Taylor et al, 2017). Further enhancing this integration, the proprietary ANTT clinical procedure guidelines (picture-based, sequenced and risk-assessed step-by-step guidance) are a tangible and accessible translation of the proprietary ANTT approach into real-world practice; these simple but effective tools have proved extremely popular, allowing the explicit description of correct utilisation of medical devices with safe aseptic practice (Rowley et al, 2010; Rowley and Clare, 2019).
Conclusion
Skin antisepsis is a critical component of PIVC insertion and maintenance. Best practice requires the use of the proprietary ANTT approach, or other type of standard aseptic technique, to achieve and maintain asepsis during the insertion and maintenance of invasive medical devices. Compared with central venous access, there continues to be a lack of appreciation of the risks associated with insertion and maintenance of PIVCs. This is especially concerning, considering the sheer volume of PIVC insertions generally, and the significant potential for incidence of complications including bacteriaemia, especially in light of the lack of surveillance for PIVC infection.
Not least, improved standard surveillance for PIVC infection would enable healthcare organisations to make more informed choices on product procurement for skin antisepsis that take into account the full cost of PIVC infection. Put differently, using unlicensed medical devices may not be cost-effective when set against the ever-increasing costs of treating bacteraemia, antimicrobial resistance, extended hospitalisations and the realities of litigation in an increasingly litigious society.
It may be prudent for healthcare organisations to fully consider the implications of a risk-register approach to using non-licensed products, especially when factoring in the lack of surveillance, the cost of treating infection, increases in length of admissions, potential litigation and reputation. At the very least, there would appear to be room for more transparency regarding keeping patients informed on the use of unlicensed products as a medicine.
Ways to improve the safety of this most common invasive procedure for patients include effective staff education regarding the risk of PIVCs, access to appropriate licensed products for skin antisepsis, and training in integrating effective skin antisepsis with best-practice the proprietary ANTT approach for PIVC insertion.

