Homeostasis can be defined as the relative stability of an organism's internal environment, regulated by negative feedback in response to internal and external stimuli (Amerman, 2016). Homeostasis encompasses a series of dynamic self-regulated processes that ensure balance through physiological adaptations. These changes are captured by a feedback system, enabling cells, tissues, and organs to compensate and restore balance (Modell et al, 2015). Nevertheless, compensatory mechanisms can sometimes fail and cause ill health.
This article considers homeostasis from a pathophysiology standpoint, examining the elements that contribute to the continuum between health and disease. It will explore homeostatic mechanisms, including the feedback loops involved in their regulation and relevant compensatory processes. A case study is provided that illustrates some of the concepts covered in this article in the context of clinical practice.
Central nervous system
The nervous and endocrine systems are tasked with regulating homeostasis. They act together to compensate and regulate signals to preserve equilibrium (Tortora and Derrickson, 2014). The endocrine system affects cellular behaviour indirectly by releasing hormones, whereas the nervous system acts directly to inhibit or excite target cells by producing action potentials, resulting in a faster response (Amerman, 2016). This pathway is regulated by the autonomic nervous system (ANS), which is underpinned by the hypothalamus.
Central and peripheral sensory information is conveyed to the hypothalamus, which co-ordinates endocrine activities and crucial brain functions essential for survival (Shahid et al, 2023). These vital processes are possible through the interaction with the pituitary gland and the nuclei in the reticular formation of the brainstem in the central nervous system (CNS) (Mangold and Das, 2023). Table 1 shows how different divisions of the ANS, namely the sympathetic and parasympathetic, activate specific centres and target cells via pre-ganglionic and post-ganglionic neurotransmitters, to produce specific effects (Amerman, 2016).
Table 1. Autonomic nervous system and homeostasis
| Stimulation | Effect on the body | Mechanism |
|---|---|---|
| Sympathetic chain ganglia |
|
Release of neurotransmitters, norepinephrine and acetylcholine on target cells |
| Parasympathetic system |
|
Release of neurotransmitter, acetylcholine on target cells |
Source: Amerman, 2016
Signals are transmitted along neurons via action potentials into (afferently) and out of (efferently) the CNS to influence target organs (Amerman, 2016) (Figure 1). The reticular formation plays a significant role as a control system within the CNS, providing essential life functions including cardiovascular, sleep, consciousness, and pain modulation. Neurotransmitters are released through the ascending reticular activating system (ARAS), and the descending pathways via the reticulospinal tract, which is mostly concerned with locomotion and postural control (Mangold and Das, 2023). These functions are linked to audio-visual and vestibular nuclei to promote proprioception. Damage affecting the reticulospinal tract portion of the brainstem may induce localised motor dysfunctions leading to paralysis, ataxia, spinal shock, and loss of respiratory drive (Mangold and Das, 2023). Damage to the ARAS and pre-frontal cortex may diminish alertness and lead to a comatose state (Mangold and Das, 2023).
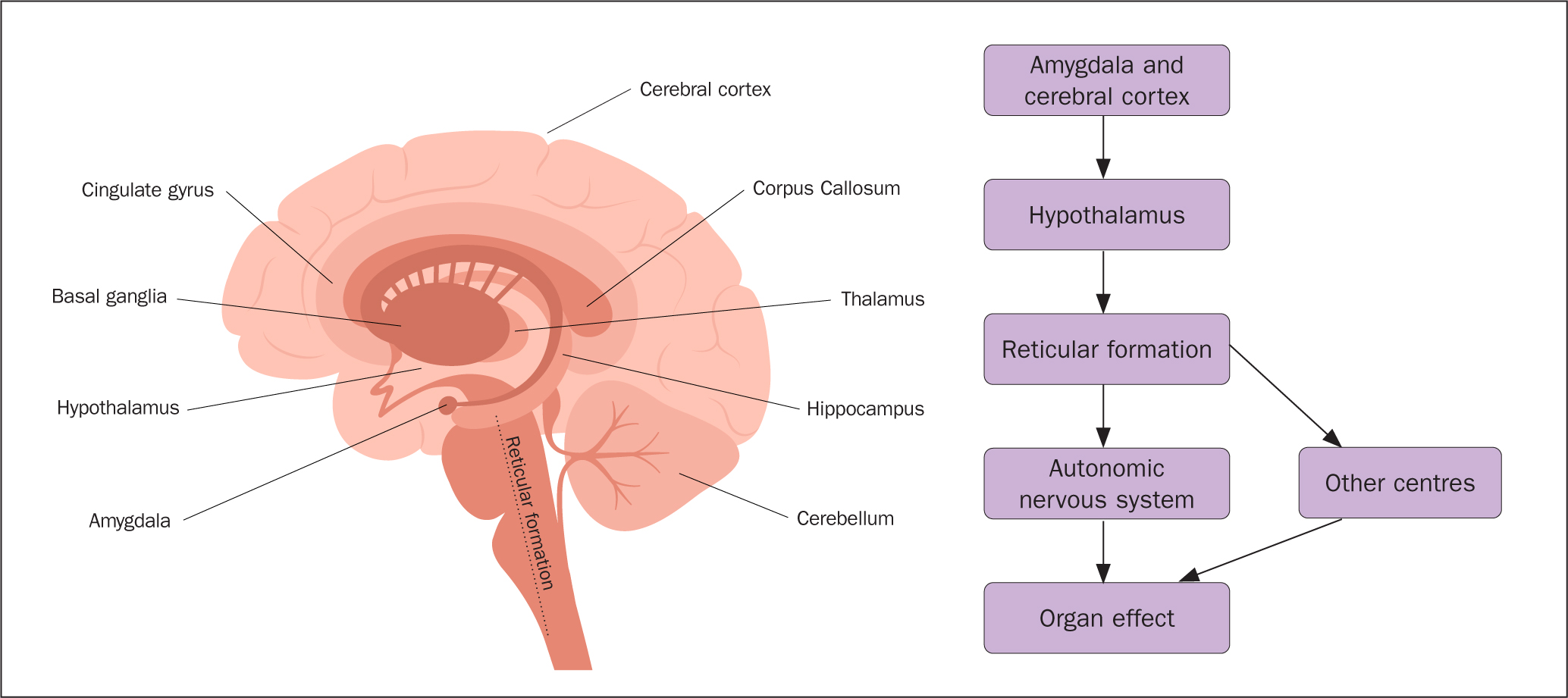
In response to internal and environmental stimuli, the body's regulatory systems maintain balance through thermoregulation, immune response, hormonal adjustments, and more (Amerman, 2016). However, prolonged exposure to stressors may compromise the body's ability to self-regulate, leading to negative health issues.
Feedback loops
Feedback mechanisms are essential components of homeostasis, as they enable the body to monitor and adjust various physiological processes to maintain internal balance (Tortora and Derrickson, 2015). There are two main feedback mechanisms: negative feedback and positive feedback.
Negative feedback is the most common feedback mechanism in homeostasis and its main objective is to modulate and restore (Torday, 2015). Deviations from physiological baseline are detected by receptors, which convey the information upwardly to a control centre in the CNS (see Table 2). The CNS elaborates the input received before communicating to effectors, cells or muscles, via afferent impulses to carry out compensatory actions (Marzvanyan and Alhawaj, 2023). As the parameter approaches the set point, the feedback loop is inhibited, preventing overshooting, and maintaining stability.
Table 2. Types of receptors
| Category | Type | Main function | Mechanism |
|---|---|---|---|
| Sensory (exteroceptors) Transduce inputs received from the external environment to the CNS | Photoreceptors | Vision | Sensory signals trigger a receptor potential that mediates release of neurotransmittersThese relay the signal to specific nerves that send impulses to the brain |
| Hair-cell receptors | Hearing and balance | ||
| Chemoreceptors | Taste and smell | ||
| Thermoreceptors | Temperature | ||
| Nociceptors | Pain | ||
| Mechanoreceptors | Pressure, vibration, texture, stretch | ||
| Sensory (interoceptors) transduce inputs received from the internal environment to the CNS | Chemoreceptors | Changes in glucose, acid–base balance | Afferent signals are transmitted to the CNS via peripheral sensory ganglia, located in:
|
| Mechanoreceptors | State of bladder, GI tract, blood pressure | ||
| Osmoreceptors | Detects osmotic pressure | ||
| Nociceptors | Visceral and referred pain | ||
| Thermoreceptors | Detects internal temperature | ||
| Proprioceptors | Locomotion and position of body relative to the environment |
CNS=central nervous system; GI=gastrointestinal
Source: Haggard and de Boer, 2014; Amerman, 2016; Marzvanyan and Alhawaj, 2023
Positive feedback is a less common mechanism in homeostasis, it functions by amplifying variations from baseline in a system rather than balancing them (Amerman, 2016). In a positive feedback loop, the initial change triggers an excitatory response, leading to furthering the gap between baseline and deviations. For example, during childbirth, the contraction of the uterine muscles triggers the release of oxytocin. Oxytocin stimulates stronger contractions, which in turn lead to more oxytocin release. This positive feedback loop continues until the baby is delivered (Libretti and Puckett, 2023).
Impaired negative feedback loop
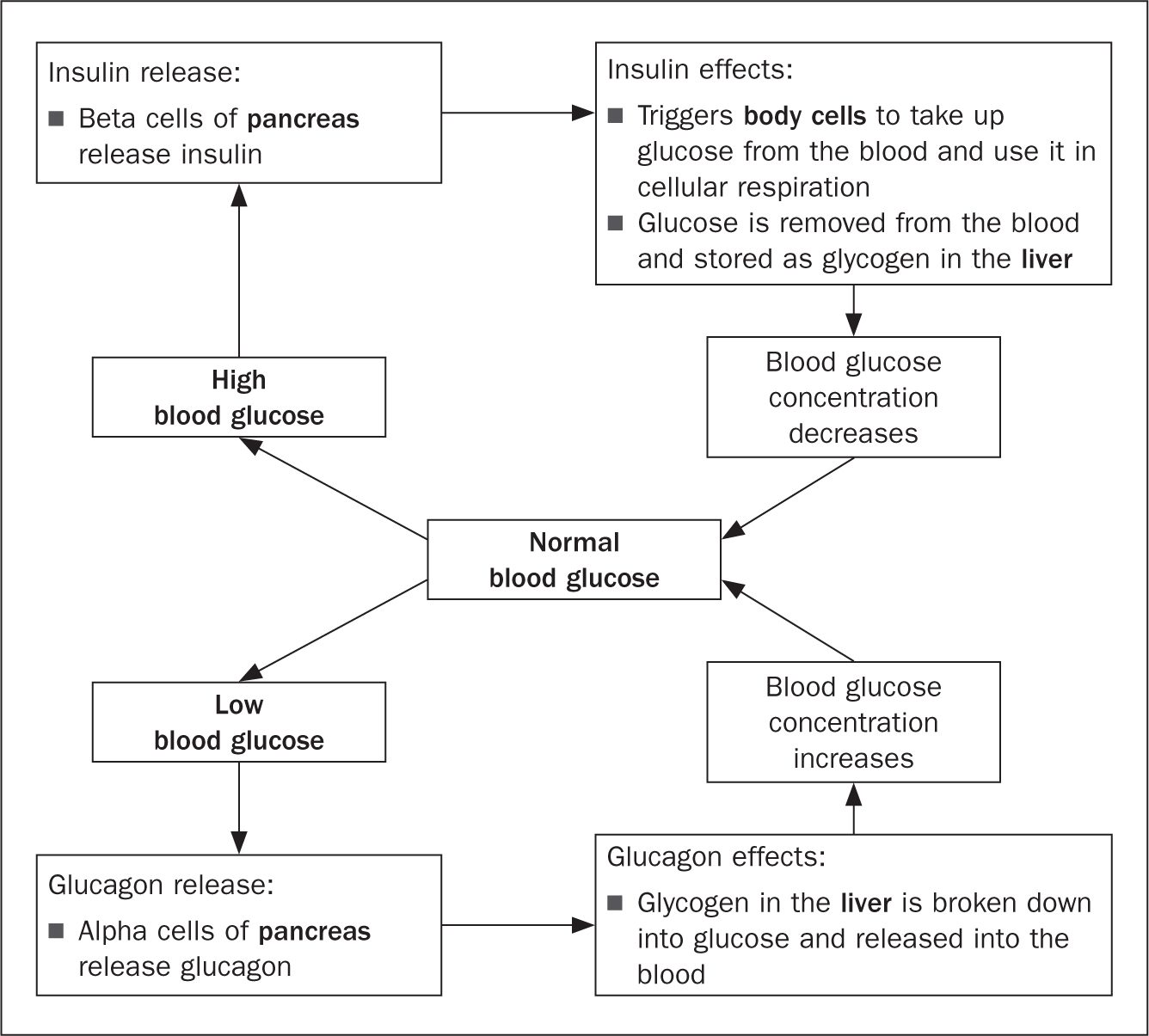
Figure 2 offers a representation of the feedback loop regulating blood glucose, which if impaired may lead to the onset of type 2 diabetes. Insulin and glucagon are released in response to variations in plasma glucose level, generated by factors such as food intake (Amerman, 2016). As food is absorbed by the small intestine, the blood glucose level rises, triggering receptors (beta cells) in the pancreas to release insulin, which in turn encourages the uptake of glucose, as a source of energy for muscle cells and for storage by promoting the conversion of glucose into glycogen (Amerman, 2016). A second negative feedback loop inhibits the production of insulin to ensure glucose levels do not exceed the set point. In the balancing act of maintaining homeostasis, glucagon is released from pancreatic alpha cells when the blood glucose level drops below the set point (Amerman, 2016), increasing the concentration of blood glucose through the breakdown of glycogen. A further negative feedback loop inhibits the production of glucagon to ensure glucose levels do not exceed the set point. When such regulatory mechanisms are impaired by genetic, environmental and behavioural causes, glucose levels remain uncontrolled, causing the pancreas to generate insufficient insulin, or increasing cellular resistance to insulin.
Temperature regulation
Temperature regulation is a crucial homeostatic mechanism required for the normal functioning of physiological processes (Tortora and Derrickson, 2015). In fact, most of the cells and enzymes are thermo-liable – sensitive to temperature changes – and may undergo denaturation, losing their biological catalytic activity (Daniel et al, 1996). Enzyme deficiencies are linked to neurological disorders and digestive issues such as lactose intolerance (Elhawary et al, 2022).
Temperature is regulated centrally by the hypothalamus and peripherally by thermo-receptors in the skin and blood vessels (Charkoudian et al, 2017). Table 3 presents the mechanisms behind temperature regulation. Other internal systems intervene in body temperature regulation. For example, the sex hormones oestradiol and progesterone induce vasomodulation resulting in heat dissipation and heat conservation, respectively (Charkoudian et al, 2017).
Table 3. Temperature regulation mechanisms
| Stimulus | Thermoregulatory response | Action | Effect |
|---|---|---|---|
| Elevated body temperature | Vasodilation | Dilated blood vessels | Increased blood flow to skin, releasing heat |
| Sweating | Activation of sweat glands | Increased sweat production favours release of moisture onto the skin's surface. As sweat evaporates, it absorbs heat from the body, cooling it down | |
| Low body temperature | Vasoconstriction | Narrowed blood vessels | Reduced blood flow to skin, conserving heat |
| Shivering | Activated muscle contractions | Shivering generates heat through increased metabolic activity |
Source: Amerman, 2016; Charkoudian et al, 2017
Disruptions in temperature regulation: fever
Infections and non-infectious triggers induce the hypothalamus to raise the internal temperature, inhibiting pathogen growth and increasing chances of survival, resulting in fever (Balli et al 2023). The central thermostat is stimulated by prostaglandins and immune-specific molecules, in response to exogenous pyrogens such as microorganisms, and endogenous pyrogens such as interferon KM (Balli et al, 2023). However, fever could also induce cell damage or death caused by hyperthermia, with disruptions to cell membranes, protein synthesis and DNA, resulting in organ failure (Walter et al, 2016).
pH balance
pH balance, also known as acid–base balance, is a critical homeostatic mechanism that ensures the optimal functioning of enzymes, proteins, and processes in the body including blood oxygenation (Amerman, 2016). The physiological pH scale ranges from 7.36 to 7.44; pH levels below 7.35 cause acidaemia, whereas alkalaemia occurs above a pH of 7.45 (Hamm et al, 2015). Acid-base homeostasis is maintained via chemical and physiological buffer systems. The former is regulated by bicarbonate ions (HCO3-), overseen by the nephrons, and the latter by respiratory and urinary systems (Amerman, 2016).
HCO3- reabsorption and production is linked to secretion of hydrogen ions (H+) via the proximal and distal tubules of the nephron in the kidney. Increased secretion of H+ would result in high reabsorption of HCO3-, resulting in alkalosis. Conversely, reduced secretion of H+ causes low reabsorption of HCO3-, causing acidosis (Hamm et al, 2015).
Although the main compensatory mechanisms are overseen by the interplay of the respiratory and renal systems (Table 4), hormones are also implicated in the regulation of acid-base balance by influencing proximal HCO3− reabsorption, (Amerman, 2016). This is stimulated primarily by adrenergic agonists, angiotensin II, aldosterone and parathyroid hormones, (Hamm et al, 2015).
Table 4. pH regulation and compensatory systems
| Compensation | Stimulus | Action | Effect |
|---|---|---|---|
| Kidneys | Decreased pCO2 (Respiratory alkalosis) | Secretion of excess H+ via Na+/H+ in distal tubule and reduced HCO3- reabsorption in proximal tubule | Retained H+ reduces systemic pH through HCO3- decreased reabsorption |
| Elevated pCO2 (Respiratory acidosis) | Secretion of excess H+ via Na+/H+ in proximal and distal tubules, HCO3- increased reabsorption and production | Increased secretion of H+ increases systemic pH through HCO3- increased reabsorption | |
| Respiratory system | Metabolic acidosis (pH below 7.36) | Chemoreceptors increase alveolar ventilation | CO2 is excreted through increased breathing rate to increase systemic pH |
| Metabolic alkalosis (pH above 7.44) | Chemoreceptors reduce alveolar ventilation | CO2 is retained through decreased breathing rate to decrease systemic pH |
Source: Amerman, 2016
Blood glucose control
Blood glucose is the primary energy source for cells, particularly in the brain and muscles (Amerman, 2016). Maintaining blood sugar within a narrow range is essential for ensuring energy availability to cells while preventing detrimental effects on various organs and systems. A negative feedback loop regulates the homeostatic mechanisms involving insulin and glucagon, which work to keep blood glucose levels stable, within a narrow range of about 4-6 mmol/litre before meals (Röder et al, 2016). This tight control is made possible by four complex axes or pathways, between the islets of Langerhans in the pancreas and the brain, the liver, the gut and the adipocytes/myocytes, summarised in Table 5 (Röder et al, 2016).
Table 5. Blood glucose regulation pathways
| Brain–islet axis | This axis relies on pancreatic parasympathetic and sympathetic innervation that lead to the autonomic nervous system, and receptors located within the hypothalamus, cerebral cortex, cerebellum and hippocampal formation. As a result, brain injuries are associated with insulin hypersecretion and higher glucagon levels. Glucagon release may also be regulated by a similar pathway whereby norepinephrine inhibits insulin secretion, notably a compensatory mechanism during the fight or flight response |
| Liver–islet axis | This axis is modulated by the liver, which releases or stores glucose based on the interaction between glucagon and insulin |
| Gut–islet axis | This axis acts in response to food intake, whereby hormones to stimulate beta cells and alpha cells are released to augment insulin and glucagon secretion. Conversely, somatostatins are produced to inhibit insulin and glucagon release |
| Adipocytes/myocytes–islet axis | This axis regulates insulin release through adipokines and myokines secreted from the adipose and muscle tissues, respectively |
Source: Roder et al, 2016
Metabolic insulin secretion by beta cells in the pancreas in response to increases in blood glucose (hyperglycaemia) facilitating the uptake of glucose by cells and its storage as glycogen in the liver and muscles (Amerman, 2016). Cephalic insulin release takes place in anticipation of a meal and before food intake, allowing a more controlled post-prandial glucose level. Naturally, glucagon is released from alpha cells in the pancreas when blood sugar is low (hypoglycaemia) triggering hepatic glycogenolysis (Amerman, 2016). The effects of uncontrolled blood glucose can be felt immediately and could be easily reversible, unless caused by underlying health conditions (Table 6). Transient hyperglycaemia could occur due to excessive food intake and manifests with excessive thirst, frequent urination, and fatigue (Röder et al, 2016). The same symptoms could occur in the initial stages of diabetes. Hyperglycaemic symptoms could lead to inflammation of nerves and blood vessels, resulting in neuropathies and vascular diseases, including myocardial infarctions, kidney failure and dementia (Amerman, 2016). Conversely, hypoglycaemia can lead to reversible symptoms such as weakness, shakiness, and confusion. However, persistent hypoglycaemia can induce muscle atrophy and diabetic coma secondary to ketoacidosis (Amerman, 2016).
Table 6. Impacts of high blood glucose on organ systems
| System | Cause | Effect |
|---|---|---|
| Cardiovascular | Vasculopathies characterised by chronic inflammation of blood vessels, which leads to reduced blood supply | Increased risk of heart disease, stroke, retinopathy, reproductive health disorders |
| Nervous | Nerve damage (neuropathies)
|
|
| Renal | Chronic kidney disease, diabetic nephropathy |
Source: Amerman, 2016
Osmoregulation and fluid balance
An example of an impairment to the homeostatic mechanisms regulating fluid balance is the development of diabetic nephropathy secondary to inflammation and blood flow restriction.
The glomeruli are central structures within the kidney responsible for filtering waste products from the blood (Amerman, 2016). Damage to the glomeruli is caused by inflammation of the capillaries surrounding them, impairing filtration and permeability and resulting in proteinuria and albuminuria – when proteins and albumin leak into the urine (Qazi et al, 2022). Kidney function is further impaired by glomerulosclerosis due to the formation of advanced glycation end products that accumulate in tissues over time, affecting the structure and function of kidney cells (Qazi et al, 2022). As a result, blood flow to the kidneys is diminished and renin released, triggering the activation of the renin-angiotensin-aldosterone system (RAAS), leading to intraglomerular and systemic hypertension (Qazi et al, 2022). Angiotensin II has been linked to ischaemic injuries because of interstitial fibrosis, RAAS-mediated vasoconstriction and inflammation caused by oxidative stress and pro-inflammatory cellular attraction (Quazi et at, 2022).
Clinical results are captured via the estimated glomerular filtration rate (eGFR), which normally measures 90 ml/minute/1.73m2 or higher, and drops to less than 15 ml/minute/1.73m2 in the development of diabetic nephropathies (National Institute for Health and Care Excellence, 2021). Over time, the cumulative effects of glomerular damage, proteinuria, inflammation, and other factors lead to diabetic nephropathy and, if untreated, to kidney failure.
Disruptions in homeostasis from environmental factors
Environmental factors can disrupt homeostasis at different levels (Table 7). Short-term exposure to some stimuli may cause transient visible changes such as increased perspiration as a response to hot weather, or colder extremities and reduced urine output in colder days. Conversely, long-term exposure may induce long-lasting changes that affect health and wellbeing. In essence, environmental factors can severely affect a person's mental health by influencing neurotransmitters, stress responses, mood regulation, and emotional experiences.
Table 7. Environmental factors and impact on homeostasis
| Factors | Impact |
|---|---|
| Psychological stress | Work-related pressures or social conflicts, could trigger hormonal responses that affect various systems. Stressful situations trigger the release of hormones such as adrenaline and cortisol, via the hypothalamic–pituitary–adrenal (HPA) axis, to prepare the body for the fight-or-flight response. Prolonged or chronic stress can lead to dysregulation of the HPA axis, impacting mood, anxiety, and overall mental wellbeing. Hormones such as cortisol and thyroid can influence the availability and sensitivity of neurotransmitter receptors, such as serotonin, dopamine, and norepinephrine, severely affecting mood regulation and emotional stability |
| Nutritional factors | Low availability of nutrient-rich food and behavioural choices predominantly tending towards nutrient-deficient food can lead to severe health complications, causing imbalances in gastrointestinal health and immune function and increasing the likelihood of developing metabolic conditions. Poor nutrition highly impacts the gut microbiota, which has been linked to modulating food absorption, mood and energy level and indirectly linked to mental health conditions such as anxiety and depression. One school of thought ascribes the latter to diets high in refined sugars and low in fibre, which cause blood sugar spikes and crashes, straining the body's ability to regulate insulin and contributing to insulin resistance, a precursor to diabetes. Other causes of inadequate nutrition include electrolyte and mineral imbalances, which affect:
|
| Noise pollution | Excessive noise pollution can disrupt sleep patterns, stress levels, and overall wellbeing by promoting cortisol release, proinflammatory responses and altering the circadian rhythms. Thus, exponentially increasing the risk of:
|
| Chemical exposure | Exposure to harmful chemicals even at low consistent dose could disrupt homeostasis and increase the risk of developing long-term conditions such as metabolic disorders, cardiovascular disease, respiratory conditions and cancer |
Source: Leontino, 2023
Case study
Presentation and history
George, a retired taxi driver, is a 65-year-old man living in London who was admitted to the accident and emergency department after developing acute pain in his lower left flank and losing consciousness at home. On presentation, George was severely hypotensive, with an oxygen saturation of 80% on 4 litres of oxygen, administered via nasal canula, and scoring 8/15 on the Glasgow Coma Scale (E:1, V:2, M:5).
George had a past medical history of kidney stones and benign prostatic hyperplasia treated medically with tamsulosin. Three weeks before this admission, George underwent a ureteric stent insertion, which became infected. As a result, while in the emergency department an emergency surgical removal of the ureteric stent took place under general anaesthetic. Following the surgery, George was admitted to the intensive care unit (ICU).
Physical examination
In the ICU George was intubated and mechanically ventilated, his arterial blood gas showed sign of uncompensated metabolic acidosis due to hypoxia (Table 8). George was pyrexic at 38.9°C and peripherally cool to touch and haemodynamically unstable, presenting with tachycardia and hypotension, despite inotropic support. Blood results indicated raised inflammatory markers and elevated white blood cell count. A Robinson drain inserted in theatre drained 60 ml of pus-like fluid.
Table 8. Case study: Arterial blood gas results
| Test | Normal results | George's results |
|---|---|---|
| pH | 7.35–7.45 | 7.24 |
| PaO2 | >10 kPa | 7.3 kPa |
| PaCO2 | 5.4 kPa | 5.4 kPa |
| HCO3- | 22–26 mmol/litre | 18.5 mmol/litre |
| BE* | -2 to +2 mEq/litre | -6.8 mEq/litre |
| Lactate | <2 mmol/litre | 6.8 mmol/litre |
| SaO2 | 94–98% | 86.8% |
| FiO2 | 21% | 55% |
Initial impression and management
Source control, the management of the root cause, is a key element in the management of sepsis. In George's case the presence of a ureteric stent due to be removed was thought to be the primary source of infection therefore it was removed.
Based on George's recent surgery and presentation, he was diagnosed with septic shock secondary to urosepsis. Sepsis is a life-threatening organ dysfunction caused by a dysregulated immune response to infection. Septic shock is defined as a ‘subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality’ (Evans et al, 2021). As a result, the management of George's condition required the Sepsis 6 care bundle (Evans et al, 2021).
Blood cultures were performed in the emergency department and in the absence of an identified causal pathogen George was administered the broad-spectrum antibiotic combination piperacillin 4 g/tazobactam 0.5 g (Tazocin) at the recommended dose of 4.5 g three times a day (Joint Formulary Committee, 2024). Despite the threat posed by multi-drug resistant organisms, the benefit of early empirical administration of broad-spectrum antibiotics in sepsis and septic shock remains a strong recommendation (Evans et al, 2021).
Crystalloid fluids were administered at 30 ml/kg, totalling 3 litres, to replace intravascular volume and improve cardiac output. However, George remained profoundly hypotensive, requiring the initiation of inotropic support to achieve a mean arterial pressure (MAP) of at least 65 mmHg. The fluid input and output were closely monitored as well as signs of ‘third spacing’ – the movement of fluid from blood vessels into the interstitial spaces of tissues, leading to reduced circulating blood volume and potential tissue oedema (Dargent et al, 2023). Hypoperfusion further enhanced hypoxia, this was seen in George's oxygen saturation of 86.8% and PaO2 of 7.3 kPa despite the high concentration of inspired oxygen (FiO2: 55%) administered via a mechanical ventilator on mixed volume-controlled mode.
Pathophysiology and clinical presentation
Pro-inflammatory cytokines, released by white blood cells, are responsible for the septic cascade resulting in pyrexia and haemodynamic instability. Pro-inflammatory cytokines encourage vasodilation and increase vascular permeability by triggering nitric oxide release from the endothelium, resulting in fluids leaking into the interstitial space. A decrease in systemic vascular resistance and cardiac output was shown in George's low blood pressure. Tachycardia of 121 beats per minute was suggestive of increased cardiac distress and compensatory mechanisms to achieve an adequate cardiac output and tissue perfusion. George was peripherally cool but centrally warm, which in the presence of high lactate was suggestive of hypoperfusion, possibly caused by peripheral vasoconstriction. Lactatemia in sepsis is the consequence of anaerobic respiration and can lead to metabolic acidosis as evident in George's arterial blood gas pH of 7.24. Lactate was monitored regularly throughout the patient's stay in ICU as per the Surviving Sepsis Campaign guidelines (Evans et al, 2021).
Summary
Homeostasis is critical to maintaining the internal balance of the human body. Key homeostatic mechanisms have been discussed including temperature regulation, pH balance, blood sugar control, electrolyte balance, and osmoregulation. These systems are regulated mostly via negative feedback loops, which counteract deviations from set points to restore equilibrium. Examples have linked homeostasis to some of the most common ailments through the lens of pathophysiology. Environmental factors and lifestyle choices have also been considered, highlighting the potential negative effect on hormonal regulation and chronic inflammation, which may lead to long-term conditions and cancer. A clinical case study has been presented to promote a deeper appreciation of homeostasis in the context of patient care, which may inform future strategies for managing and preventing illness.
KEY POINTS
- Homeostasis involves the body's regulatory mechanisms ensuring balance in response to internal and external stimuli – if these mechanisms are compromised it can result in ill health
- Fever is a natural response triggered by infections or non-infectious stimuli and regulated by the hypothalamus via prostaglandins and pyrogens. However, excessive fever can cause cellular damage and affect DNA, potentially leading to organ failure
- Temperature regulation and pH balance are interconnected, as metabolic activity, respiratory changes, and other physiological responses to temperature shifts can influence acid–base equilibrium
- Unregulated blood sugar disrupts homeostasis and damages organ systems, particularly the kidneys, where sustained hyperglycaemia can lead to diabetic nephropathy, impaired filtration, and progressive renal failure
- The clinical case study here shows the effects of disrupted homeostasis in the context of patient care, where septic shock induced haemodynamic instability, hypotension, hypoperfusion and subsequent hypoxia, caused by pro-inflammatory cytokines
CPD reflective questions
- Considering the effects of stress and poor nutrition on homeostasis, what can you do to enhance your resilience and improve your wellbeing?
- How can understanding the interconnected mechanisms of homeostasis, such as pH balance, temperature regulation, and blood sugar control, help you anticipate and intervene in early signs of patient deterioration?
- Considering the case study, how will your ability to detect and respond to homeostatic imbalances, such as fever or electrolyte disturbances, influence patient outcomes?


