Medical devices designed to access the vascular system for the purpose of infusion therapy date back as early as the mid-1600s when Christopher Wren, a renowned anatomist at Oxford University, used a quill and a pig's bladder to create the first working intravenous infusion device. Little progress was made in the field until 1818 when Dr Blundell performed the first successful human-to-human transfusion using a what he termed a ‘Gravitator.’ This device was composed of a syringe attached to a tube which was attached to a funnel. The donor would bleed into the funnel and the blood would, via gravity, travel down the tube and into patient. This approach was ‘to be used when all other options were exhausted’ and did not have a very high success rate; however, it proved that infusions into the bloodstream could serve as a viable method for improving patient outcomes. While intravenous infusions increased in frequency of use during the late 1800s and early 1900s, it was not until in 1950 that a quantum leap forward occurred when Dr David Massa, a resident in anesthesiology, fitted a polyvinyl chloride catheter over a steel introducer needle, creating the first catheter that could be directly threaded into a vessel after percutaneous venipuncture with a needle.1 This discovery sparked an instant revolution in healthcare and forever changed the way intravenous infusions were delivered. In the more than 70 years that followed, Massa's over-the-needle (OTN) catheter configuration inspired a multitude of innovative peripheral intravenous catheter (PIVC) devices. Iterative improvements in catheter materials, patient comfort, blood control, and needle stick safety contributed to making catheter OTN PIVCs the primary vehicle for delivering life-saving intravenous infusions to upwards of 90% of hospitalized patients.2
PIVCs now account for the majority of vascular access devices used in health care with an estimated 330 million placed annually in the United States alone.3 Though PIVCs have indeed become commonplace in health care and are considered generally safe, they do come with certain risks. Common PIVC risks include subsequent swelling of the vein known as phlebitis, inadvertent leaking of the infusion into the surrounding soft tissue known as an infiltration or extravasation, poor first stick insertion success rates, and a high rate of failure prior to completion of therapy.4–9 However, one of the major risks related to PIVC use is blood stream infection (BSI).10 A PIVC-related BSI can be defined as the presence of a BSI that has originated from the PIVC. Most often, a BSI is verified by a positive semiquantitative catheter culture (≥15 colony forming units [CFUs]) where the same organism is cultured within the blood.11 Published research shows PIVC-related BSI rates range from 0.1% to 1.4%.12–14 While these studies have variability in their PIVC-related BSI rates, all concluded that by absolute prevalence PIVCs account for the majority of hospital-acquired catheter-related BSIs. Moreover, these rates may in fact be much greater because formal surveillance and reporting of PIVC-related BSI is not mandated and therefore falls short of routine.
It is well understood percutaneous, non-tunneled vascular access devices, including PIVCs, may be contaminated in two specific ways. First, contamination may occur during insertion because of the exposure of the catheter's external surface to the skin. This allows bacteria to adhere to the catheter and form microcolonies that can ultimately grow large enough to detach into the blood stream to cause sepsis.15,16 Second, contamination may occur through the intraluminal pathway. This can occur through poor PIVC care and maintenance, poor disinfection of needleless adaptors, or a contaminated infusate. In a study by Safdar and Maki, it was revealed that in short-dwelling catheters (<14 days), extraluminal colonization from skin microorganisms along the outer surface of the catheter predominates as the pathomechanism of infection.17 Further research indicated catheter-related BSIs associated with percutaneously placed non-tunneled catheters that occur within the first 10 days of insertion are most often correlated with extraluminal contamination.18–20 These observations align well with Helm et al., who clearly states that extraluminal and intraluminal contamination have different pathogenic mechanisms and temporal characteristics. Helm et al shows that extraluminal contamination occurs early and intraluminal contamination appears later in the catheter's dwell time.2 Mermel echoes this sentiment when he writes, ‘Most of the evidence suggests that, in general, an extraluminal source of infection predominates in catheters placed for a shorter duration of time, whereas an intraluminal source predominates with more prolonged dwell times.’21 Furthermore, Elliott et al. cultured the tips of percutaneous, non-tunneled vascular access devices placed over a wire during cardiac surgery and discovered 16% of the catheter tips were colonized within 90 minutes of catheter placement.22 With the majority of PIVC insertions occurring outside the surgical theater, one could surmise that PIVC contamination potential is substantially higher. Coupled with the information above and the fact that the average length of stay in an acute health care facility is 4.6 days, we can deduce early extraluminal contamination of PIVCs represent a major threat to many hospitalized patients.23
To better understand the mechanism of early extraluminal catheter contamination, it is crucial to understand the physiology and flora of human skin. The skin is normally colonized by millions of bacteria existing in a microbiome known as skin flora. Approximately 80% of this flora may be found within the first 5 cell layers of the stratum corneum, which is the outermost layer of the skin. The remaining 20% live within the underlying epidermis, sebaceous glands, and hair follicles.24,25 These areas cannot be disinfected by the current standard of skin preparation prior to the placement of a PIVC.26–29 It has been demonstrated this bacterial skin flora has a significant potential to colonize a PIVC.30,31
Staphylococcus aureus (S. aureus) and coagulase negative staphylococci are among the most common flora resident on and within the skin and are found in CFUs.32 A single staphylococcus CFU is comprised of 200,000 to 400,000 individual bacteria, and a single CFU of staphylococci has been found to have a doubling time as rapid as 28.8 minutes at 37°C.33,34 Using this formula, it is conceivable a single CFU transferred to a catheter surface during insertion can replicate and grow to reach the defined threshold for catheter colonization (≥15 CFU) in less than 2 hours.
In this study, we propose the novel use of a physical barrier that could serve to protect the catheter from harmful flora as it is advanced through the skin during insertion. The objective of the present ovine study is to determine whether deploying a novel through-the-needle (TTN) catheter, which is protected from skin flora, reduces catheter contamination when compared to the traditional OTN configuration, where the catheter makes direct contact with the skin during insertion.
Materials and methods
In order to study the effects of using a novel TTN device, which deploys a 20-gauge catheter through a needle (Figure 1), compared to a more traditional OTN device, which deploys a 20-gauge catheter over a needle, we used 3 healthy sheep weighing 40 kg to 60 kg for the experimental procedures. Sheep were used to best reflect the dynamic interactions between the various tissues. Additionally, sheep are a common model for vascular access because of similarities in vascular anatomy and hemodynamics. Castrated male sheep (wethers) and female sheep (ewes) were selected; however, the animals' sex would have had no impact on the results of this study.

Insertion sites (total of 10 insertion sites) including cephalic, saphenous, and jugular sites were cleaned with soap and water, shaved, then dried. An approximate 1.5 in by 1.5 in zone around the preferred access site on the newly cleaned skin of the wether or ewe was marked using a surgical marker, then 1 mL aliquot suspension of approximately 1 × 108 CFU of S. aureus (ATCC 29213, Manassas, VA) in tryptic soy broth TSB was applied by syringe within the marked zone and allowed to dry for 10 minutes in order to introduce equal levels of known bacteria to the access site on the skin of the wether or ewe.
Five experimental TTN devices (OspreyIV 20 g Skydance Vascular, Pleasant Grove, UT,) and 5 comparison OTN devices (Insyte Autoguard 20 g Becton Dickinson, Franklin Lakes, NJ) were used for PIVC insertion throughout the 10 sites. Immediately after catheter placement, the skin within the 1.5 in marked square box was swabbed for culture to confirm that the S. aureus microorganisms were viable when the catheters were placed. After the culture swab, the 1.5 in by 1.5 in zone where the bacteria were applied was disinfected with a 70% alcohol solution in order to prevent any skin surface bacteria to be transferred to the intravenous segment of the catheter. After application of the 70% alcohol solution, the skin was then allowed to dry for at least 1 minute.
The catheters and associated tissues were then aseptically retrieved via en-bloc dissection and transferred to a sterile container. Each study catheter was aseptically removed from the surrounding tissue and segmented into 2 samples – 1 cm of the catheters' distal tip and the remaining subcutaneous catheter shaft. To shed adhered bacteria that had attached to each catheter segment, each collected sample was exposed to 2 consecutive shedding events in order to gather the maximum number of bacteria from each catheter segment. First, each catheter segment was vortexed in individual collection containers with saline + 0.5% Tween-80 for 15 seconds, followed by sonication for 5 minutes. The catheter segments were then transferred into new sterile containers with fresh saline + 0.5% Tween-80 and the original first solution was retained for bacterial analysis. The second collection containers with the catheters and saline +0.5% Tween-80, were vortexed for 15 seconds, followed by sonication for 5 minutes, providing a second bacterial solution. The 2 sonicate solutions were transferred into separate sterile tubes providing 40 samples. Sterile saline was used to prepare 101, 102, and 103 dilutions of each sample. Streaks of 100 μL of the undiluted solutions and the 101, 102, and 103 dilutions solution, in triplicate, were transferred onto Tryptic Soy Agar plates and cultured for 24 to 72 hours at approximately 37°C. To summarize the above procedure, the 10 catheters were cut into 2 segments (distal tip and remaining subcutaneous shaft), and each of the 20 segments was exposed to 2 shedding events, resulting in a total of 40 sonicate samples that were cultured on Tryptic Soy Agar plates for 24 to 72 hours.
Culture plates were defined as positive if any detectable bacteria colony growth (≥1 CFU) was visually observed. Culture plates were defined as negative if zero bacterial colony growth was observed. Any plates with >300 colonies per plate were marked as positive and too numerous to count. Statistical analysis was performed with the JMP statistical software version 16.2 (JMP is a subsidiary of SAS Institute, Cary, NC). P values < 0.05 were considered statistically significant. Binary logistic regression analysis was performed. All experimental procedures and animal care in the present study have been approved and conducted in accordance with American Preclinical Services (APS), North American Science Associates (NAMSA) policies with a study ID of: KKL001-IS75.
Results
Of the 5 OTN catheters, 2 were positive for bacterial contamination. In comparison, 0 contamination was found in the 5TTN catheters (Figure 2). Final culture results of the 20 sonicate samples gathered from the 5 TTN catheters were negative for bacterial growth. Zero bacterial colonies were found any of the samples from the distal tip segment or subcutaneous shaft segments of the TTN catheters. (Table 1). Final culture results of the 5 OTN sonicate samples revealed 0 contamination of the tip segments, and 2 out of 5 shaft segments were contaminated with measurable bacterial colonies (Table 2).
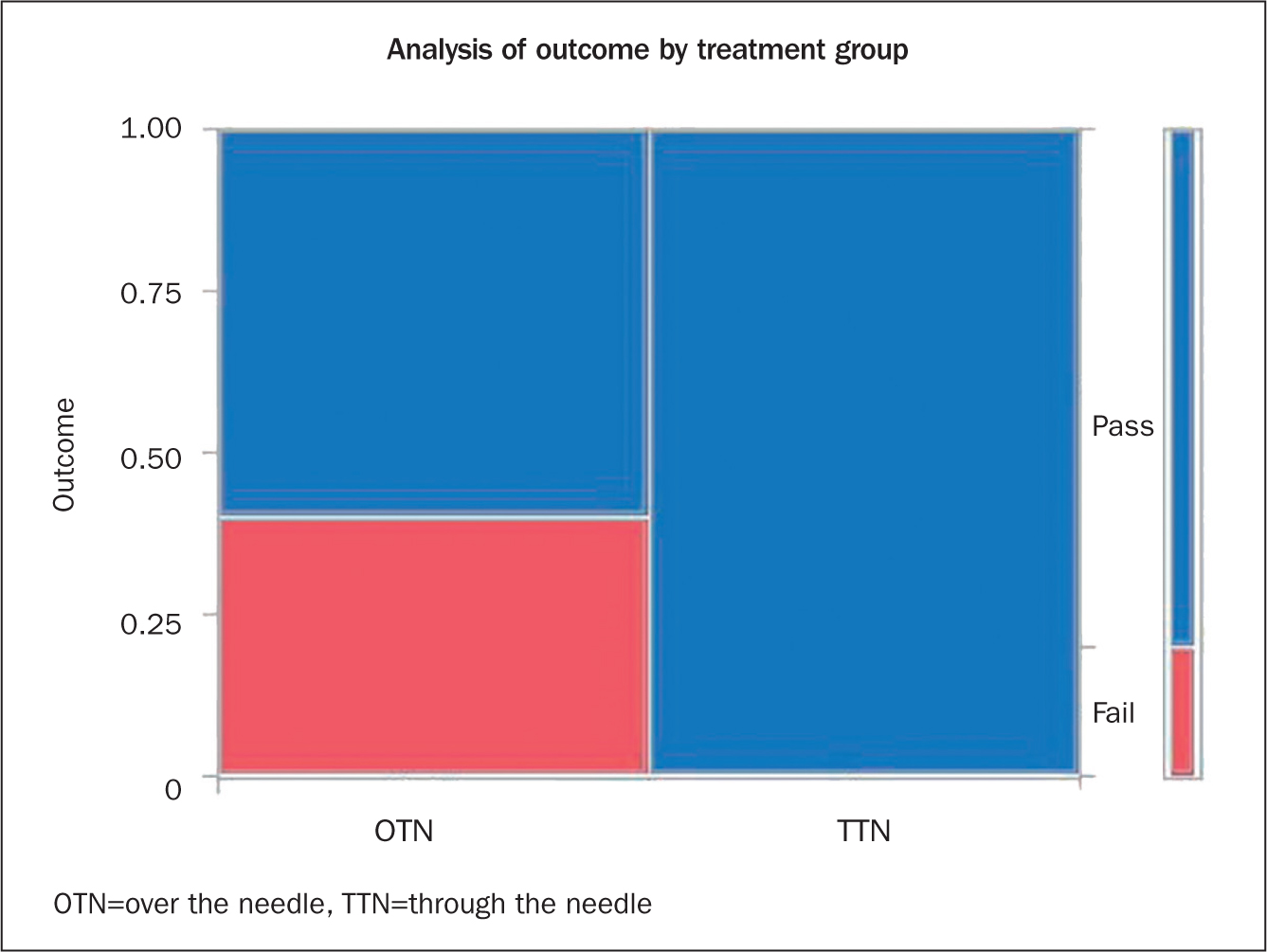
Table 1. TTN catheter
| TTN catheter test | Anatomical insertion location | Tip segment CFU, sonicate 1/sonicate 2a | Shaft segment CFU, sonicate 1/sonicate 2a | Skin swab |
|---|---|---|---|---|
| 1 | Right jugular | 0/0 | 0/0 | TNTC |
| 2 | Left cephalic | 0/0 | 0/0 | TNTC |
| 3 | Right jugular | 0/0 | 0/0 | 32 |
| 4 | Left jugular | 0/0 | 0/0 | 52 |
| 5 | Right jugular | 0/0 | 0/0 | 12 |
CFU=colony-forming units per mL, TNTC=too numerous to count (>300 CFU), TTN=through the needle
aSonicate groups include all dilutions and all replicates
Table 2. OTN catheter
| OTN catheter comp | Anatomical insertion location | Tip segment CFU, sonicate 1/sonicate 2a | Shaft segment CFU, sonicate 1/sonicate 2a | Skin swab |
|---|---|---|---|---|
| 1 | Left cephalic | 0/0 | 0/1 | 32 |
| 2 | Right saphenous | 0/0 | 0/0 | TNTC |
| 3 | Right cephalic | 0/0 | 0/0 | 42 |
| 4 | Left saphenous | 0/0 | 0/1 | 34 |
| 5 | Right cephalic | 0/0 | 0/0 | 73 |
CFU=colony-forming units per mL, OTN=over the needle, TNTC=too numerous to count (> 300 CFU)
aSonicate groups include all dilutions and all replicates
Discussion
In this present ovine study, the data revealed that use of a novel TTN approach resulted in less contamination than the more traditional OTN approach. The TTN catheter deployment resulted in 0 contamination while the OTN catheter deployment resulted in 2 out of 5 PIVCs being contaminated. The absolute risk reduction is 40%, or a 40% rate of contamination drops to a 0% rate of contamination when the TTN catheter deployment was used. The relative risk, also called the risk ratio, is defined as the risk or probability of an event (catheter contamination) relative to the independent variable, that is, device selection (OTN vs. TTN). A relative risk of 1.67 indicates that catheters placed using the OTN deployment were 1.67 times more likely to be contaminated during the insertion process.
Although the present study was limited and did not reach a 95% confidence level (P > 0.05, the P value in this study is 0.07, producing a confidence level of 93%), it does suggest that if the catheter is protected from contact with the skin, the risk of contamination is reduced or eliminated. Additionally, this study exposed a consistency of results when compared to previous studies by Livesley et al and Elliott et al that shared some similar objectives.22,35
Livesley et al compared the extraluminal catheter contamination rate of 30 central venous catheters (CVCs) inserted directly through the skin over a wire to 30 CVCs inserted through a sheath. In comparison, the CVCs inserted over a wire revealed a 17% rate of contamination versus a 3% rate of skin contamination for the CVCs placed through a sheath. These results represent an 82% reduction in contamination rates when the catheter is protected during insertion.35
Elliott et al. conducted a similar study investigating catheter contamination from unprotected catheter tip samples and reported a contamination rate of 17%. Like the present study, the CFUs recovered on their culture plates numbered between 1 and 7 CFUs.22 The present investigation shows a consistency of results with the above studies and reveals that there is a reduction in contamination when using some embodiment of catheter protection or physical barrier.
Conclusion
The cumulative data referenced within this manuscript suggests the first bacterial insult to a vascular access device is from bacteria within the skin that contacts the catheter during the insertion of the device itself. This contamination occurs despite best efforts to prepare the skin prior to the insertion procedure because resident bacteria within the deeper layers survives topical disinfection. Traditional OTN devices, developed over 70 years ago, are at inherent risk of insertion-related contact contamination. This contamination may lead to rapid catheter colonization and subsequent PIVC-related BSI potential.
Mitigation of catheter-to-skin contact during insertion has been shown to reduce contamination in a previous study. While additional research on the effects of TTN peripheral vascular access is required, the results of the present study are meaningful and consistent with prior research. Moreover, if considered alongside the accepted knowledge of persistent bacterium within the skin, as well as the known mechanism of extraluminal contamination during insertion, and the high rate of PIVC colonization, these results become suggestive of a reduction in insertion-related catheter contamination from skin when a TTN PIVC insertion method is implemented.
Vascular access clinicians have long adopted key part protection strategies such as the use of gloves, gowns, drapes, and caps to act as physical barriers for preventing contamination during many vascular access procedures.27 The effectiveness of these protective strategies is well established; however, extending such protection to the catheter itself during insertion has been largely unaddressed. This study aims to extend that similar protection to the catheter itself by investigating the efficacy of a novel TTN PIVC approach. We have shown that using a TTN approach decreases the rate of contamination and the potential for subsequent early colonization. The vascular access industry has acknowledged the inherent issue of skin-related catheter contamination, though the response has primarily focused on novel catheter materials, coatings, and impregnations.26,36 These solutions attempt to inhibit the adherence or growth of the bacteria that is inevitably encountered within the skin during insertion. The results of this research, as well as previously published studies, point toward considering physical catheter protection strategies such as TTN technologies as a potential alternative.
Editor note
indicates that continuing education contact hours are available for this activity. Earn the contact hours by reading this article and completing the test available in the AVA Online Store.
Go to https://www.avainfo.org/store/ for the CE quiz. It is free to AVA members and log in is required. It is available to non-members for $25 USD. Please use the same link and create a guest account.


